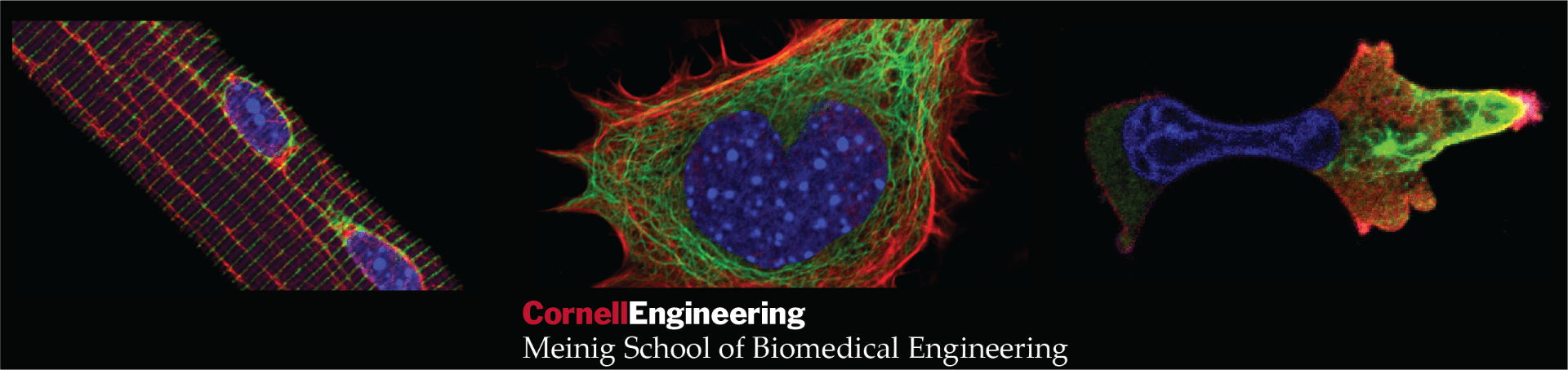
Welcome to the Lammerding Lab!
What squishing cells can tell us about human disease – studying the interplay between cellular structure, mechanics, and function.
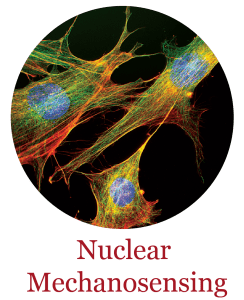
Cells in the human body reside in a physically stressful and demanding environment, being continuously exposed to large forces and deformations. Cells sense these mechanical cues and respond by performing functions critical to tissue development and maintenance. This interplay between physical forces and cellular function is now recognized as a rapidly growing area of research termed “mechanobiology”. Disturbances in mechanobiological processes can cause numerous human diseases, ranging from developmental defects to muscular dystrophy, heart disease, and cancer. Our laboratory has pioneered research into the mechanobiology of the cell nucleus, including determining the mechanical properties of the cell nucleus, understanding how the cell nucleus can sense mechanical stimuli (“nuclear mechanotransduction”), and defining the role of nuclear mechanobiology in human diseases (See selected publications).
The Lammerding Lab is a diverse interdisciplinary team using approaches that combine engineering principles, microfabrication, cell and molecular biology techniques, and novel experimental assays to investigate the intricate interplay between cellular structure, mechanics and function. Our research topics, which have extensive cross-talk and synergies, are broadly categorized below:
Selected Publications
Odell J, Lammerding J. Lamins as structural nuclear elements through evolution. Curr Opin Cell Biol. 2023. 85:102267
Kalukula Y, Stephens AD, Lammerding J*, Gabriele S*. Mechanics and functional consequences of nuclear deformations. Nature Reviews Molecular Cell Biology. 2022. 23(2): 583-602. *, denotes co-corresponding authors.
Bell ES, Shah P, Zuela-Sopilniak N, Kim D, Varlet AA, Morival LP, McGregor AL, Isermann P, Davidson PM, Elacqua JJ, Lakins JN, Vahdat L, Weaver WM, Smolka MB, Span PN, Lammerding J. Low lamin A levels enhance confined cell migration and metastatic capacity in breast cancer. Oncogene. 2022. 41(36): 4211-4230
Shah P, Hobson CM, Cheng S, Colville MJ, Paszek MJ, Superfine R, Lammerding, J. Nuclear Deformation Causes DNA Damage by Increasing Replication Stress. Current Biology. 2021. 31(4):753-765
Earle AJ, Kirby TJ, Fedorchak GR, Isermann P, Patel J, Iruvanti S, Moore SA, Bonne G, Wallrath LL, Lammerding J. Mutant lamins cause nuclear envelope rupture and DNA damage in skeletal muscle cells. Nature Materials. 2020. 19: 464-473
Kirby TJ, Lammerding J. Emerging views of the nucleus as a cellular mechanosensor. Nat Cell Biol. 2018. 20(4): 373-381
Denais CM, Gilbert RM, Isermann P, McGregor AL, te Lindert M, Weigelin B, Davidson PM, Friedl P, Wolf K, Lammerding J. Nuclear envelope rupture and repair during cancer cell migration. Science. 2016. 352(6283): 353-358
Davidson PM, Sliz J, Isermann P, Denais C, Lammerding J. Design of a microfluidic device to quantify intra-nuclear deformation during cell migration through confining environments. Integr Biol (Camb). 2015. 30(7): 1534-46
Ho CY, Jaalouk DE, Vartiainen MK, Lammerding J. Lamin A/C and emerin regulate MKL1/SRF activity by modulating actin dynamics. Nature 2013. 497(7450):507-11
Our lab remains successful due to the past and present contributions of the following funding agencies: