Cell Migration & Metastasis
Understanding the role of nuclear mechanobiology and metabolism in cell migration in confined 3-D environments.
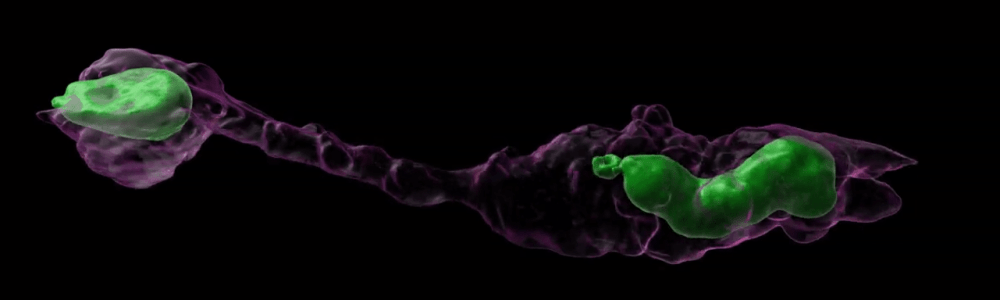
Cancer metastasis, i.e., the spreading of tumor cells into surrounding tissues and distant organs, is the major cause of cancer lethality. In the process, tumor cells must transverse interstitial spaces and openings in the vascular endothelial cell layer that are substantially smaller than the size of the cell. In a physiological context, immune cells have impressive capabilities to rapidly move from the bloodstream into tissues and migrate to sites of infection and inflammation. It is now emerging that in these processes, the deformability of the cell nucleus, the largest and stiffest organelle, can become rate-limiting for the ability of cells to move through confined spaces. Conversely, the mechanical stress placed on the nucleus during such confined migration can alter nuclear structure, integrity, and function.
Current projects:
We are working to identify the precise molecular mechanism cells use to move their nucleus through confined spaces, including the metabolic cost of this process and the cells’ ability to dynamically switch between different migration modes. As part of this work, we have designed a fluorescent biosensor to measure intracellular ATP/ADP ratios, which enables us to assess metabolic activity during confined migration in single cells. Our goal is to identify characteristic features of cells with high invasive potential that can be exploited for prognostic and therapeutic applications. In addition, we are focused on determining the short- and long-term consequences of the mechanical stress on the nucleus, including chromatin organization, gene expression, and genomic instability. At the same time, we are expanding our work to non-tumor cells, including fibroblasts and immune cells, which play important roles both in cancer progression and during physiological processes.
Key findings:
We demonstrated that the deformability of the nucleus, governed by the levels of lamin A/C, presents a rate-limiting factor in the ability of cells to transit through narrow constrictions in 3-D environments.
We showed that the unique nuclear envelope structure and composition of neutrophils, which have low levels of lamins and high levels of lamin B receptor (LBR), enhances the cells’ ability to pass through tight spaces.
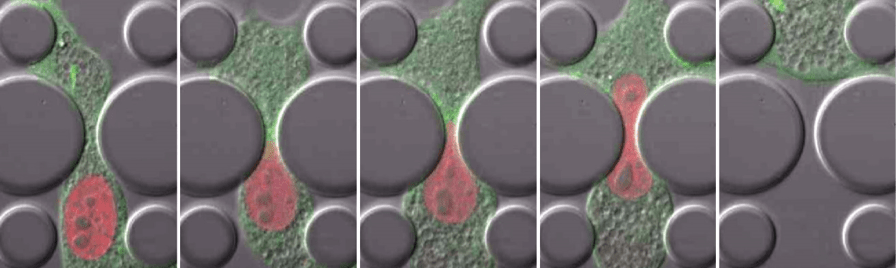
We found that nuclear deformation during confined migration leads to transient nuclear envelope rupture in vitro and in vivo, and that cells use the endosomal sorting complexes required for transport (ESCRT) machinery to restore nuclear envelope integrity.
We showed that the nuclear deformation and nuclear envelope rupture during confined migration can result in DNA damage.
We determined that nuclear deformation during confined migration or physical compression increased replication stress, thereby identifying a novel mechanism by which physical stress can cause DNA damage.
Representative publications:
Shah et al. Curr Biol 2020. Nuclear Deformation Causes DNA Damage by Increasing Replication Stress.
Mistriotis et al. JCB 2019. Confinement hinders motility by inducing RhoA-mediated nuclear influx, volume expansion, and blebbing.
Bakhoum et al. Nature 2018. Chromosomal instability drives metastasis through a cytosolic DNA response.
Denais et al. Science 2016. Nuclear envelope rupture and repair during cancer cell migration.
Davidson et al. Integr. Biol 2015. Design of a microfluidic device to quantify intra-nuclear deformation during cell migration through confining environments.
Current project team members: