Nuclear Mechanosensing
Elucidating how cells ‘sense’ and respond to their physical environment.
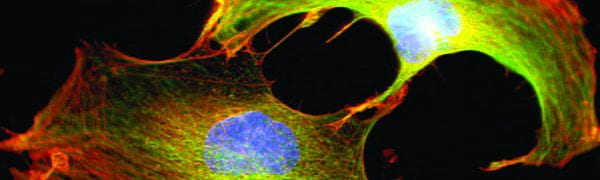
Virtually all cells respond to mechanical stimuli with the activation of specific signaling pathways and induction of mechanosensitive genes. This ‘mechanotransduction’ response enables cells to adjust to their constantly changing physical environment. Examples include muscle growth (hypertrophy) in response to exercise, adaptation of bone density to mechanical load, tissue development, and even the interaction/communication between immune cells. While many of the signaling pathways activated by mechanical stimuli are well characterized, the underlying molecular ‘mechanosensors’ that are responsible for transducing mechanical input into biochemical responses to initiate the specific signaling pathways remain incompletely understood. Our research focus is on the role of the cell nucleus and its connection with the surrounding cytoskeleton in this process.
Current projects:
We have previously shown that nuclear envelope proteins are crucial for the activation of mechanoresponsive genes. We are now investigating whether the impaired gene expression response is due to a role of nuclear envelope proteins in directly sensing mechanical forces, transmitting forces to the nuclear interior, or modifying biochemical signals from the cytoplasm. In addition, we are determining how physical forces on the cell nucleus can cause changes in chromatin organization and modifications, which could further modulate gene expression and the physical properties of the nucleus. Lastly, we are examining the evolutionary aspects of nuclear mechanobiology, including the intriguing hypothesis that lamins, which are found only in metazoan organisms, evolved to sustain the higher mechanical forces experience in animal tissues.
Key findings:
Our findings that the nuclear envelope proteins lamins A/C and emerin are required for the activation of mechanosensitive genes in vitro and in vivo provided the first evidence for nuclear mechanotransduction.
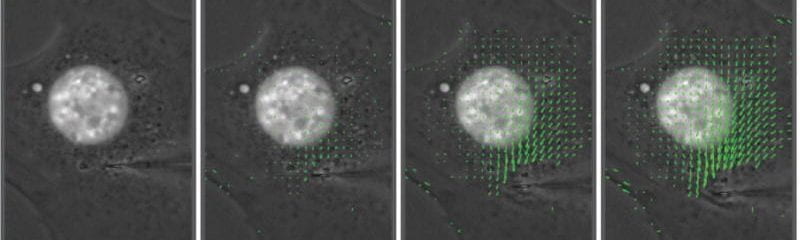
We produced the first direct measurements that the Linker of Nucleoskeleton and Cytoskeleton (LINC) complex is critical for force transmission between the nucleus and cytoskeleton, and that disrupting the LINC complex results in disturbed cytoskeletal organization and impaired cell polarization and migration.
We showed that loss of the nuclear envelope proteins lamin A/C or emerin impairs activation and nuclear translocation of the mechanoresponsive transcriptional regulator MKL1 (also known as MRTF-A) by altering nuclear and cytoplasmic actin organization.
We demonstrated that specific lamin isoforms, which are the major component of the nuclear lamina, have distinct roles in cellular mechanics, despite their similar structure and distribution.
Representative publications:
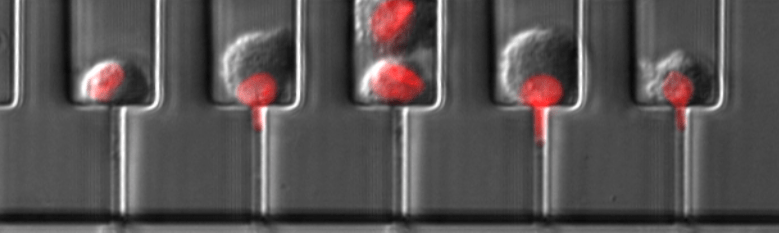
Davidson et al. Lab Chip 2019. High-throughput microfluidic micropipette aspiration device to probe time-scale dependent nuclear mechanics in intact cells.
Kirby & Lammerding. Nat Cell Bio 2018. Emerging views of the nucleus as a cellular mechanosensor.
Ho et al. Nature 2013. Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics.
Lombardi et al. J Biol Chem 2011. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton.
Lammerding et al. J Clin Invest 2004. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction.
Current project team members: