The current research efforts in the Lammerding laboratory can be broadly classified into the following three areas, although substantial cross-talk and synergies exist between these topics:
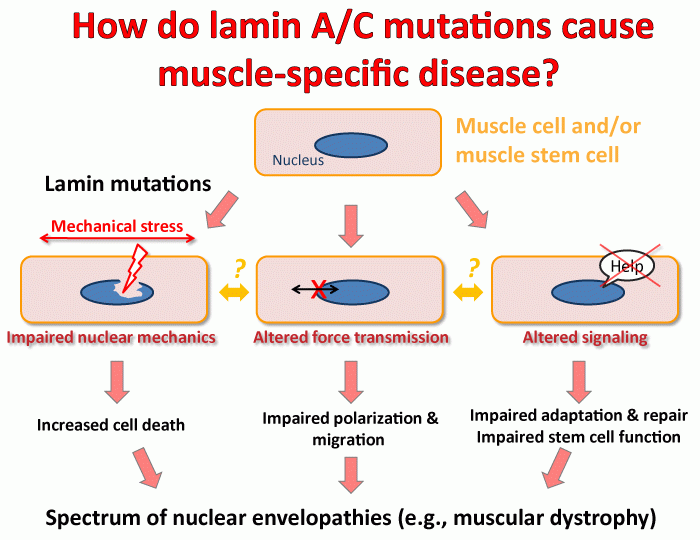
- Identifying the molecular mechanisms underlying muscular dystrophy and dilated cardiomyopathy caused by mutations in the nuclear envelope proteins lamin A/C, emerin, nesprins, and SUN proteins. Although lamins A and C are expressed in almost all differentiated cells, the vast majority of mutations cause very tissue-specific defects in patients, primarily affecting skeletal and cardiac muscle and tendons.
We are investigating three non-mutually exclusive hypotheses, namely that these diseases arise from (i) physical damage to more fragile cells/nuclei, (ii) impaired activation of mechanotransduction pathways required for normal cellular homeostasis, and/or (iii) insufficient regeneration/repair of damaged tissue due to defects in adult stem cell function and maintenance. Experimental tools include patient-derived induced pluripotent stem cells (iPSCs) that can be differentiated into cardiac myocytes and other lineages, mouse models of the diverse laminopathies, and in vitro experiments, including live-cell microscopy and cell and molecular biology approaches. These approaches let us investigate the effect of specific mutations on cellular structure and function, while also teaching us more about the normal function of these proteins. An improved understanding of the molecular mechanisms underlying the currently incurable laminopathies will be crucial to develop novel therapeutic approaches to treat or even cure these devastating diseases.
- Understanding the role of nuclear and cytoskeletal mechanics and metabolism in cell migration in confined 3-D environments.
Cancer metastasis, i.e., the spreading of tumor cells into surrounding tissues and distant organs, as the major cause of cancer lethality. In the process, tumor cells must transverse interstitial spaces and openings in the vascular endothelial cell layer that are substantially smaller than the size of the cell. In a physiological context, immune cells have impressive capabilities to rapidly move from the blood stream into tissues and migrate to sites of infection and inflammation. It is now emerging that in these processes, the deformability of the cell nucleus, the largest and stiffest organelle, can become rate-limiting for the ability of cells to move through confined spaces. We are researching how cells are able to move their nucleus through such confined spaces, what the metabolic costs are of confined migration, and whether highly metastatic cancer cells have developed specific mechanisms for confined migration that could be targeted in novel therapeutic approaches. At the same time, we are investigating the functional and genomic consequences of the large nuclear deformation associated with confined migration. We have already demonstrated that the physical stress exerted on the nucleus during confined migration can result in transient nuclear envelope rupture, DNA damage, and nuclear fragmentation. We are now investigating the molecular mechanism responsible for the DNA damage, the effect of repetitive migration through tight spaces on genomic instability, and how confined migration can lead to changes in chromatin organization and gene expression. Towards these goals, we are combining microfabrication techniques to create physiologically relevant environments in vitro, fluorescent reporters for DNA damage, metabolic activity, cytoskeletal dynamics, and nuclear envelope rupture, along with advanced live-cell imaging and and molecular biology approaches such as ATAC-seq, RNA-seq, and Hi-C. In vitro and in vivo experiments are complemented with the analysis of patient-derived samples. Insights from these study may help to identify particularly invasive cancer cells and to develop novel therapeutic approaches specifically targeting metastatic cancer cells, or to improve immune cell function.
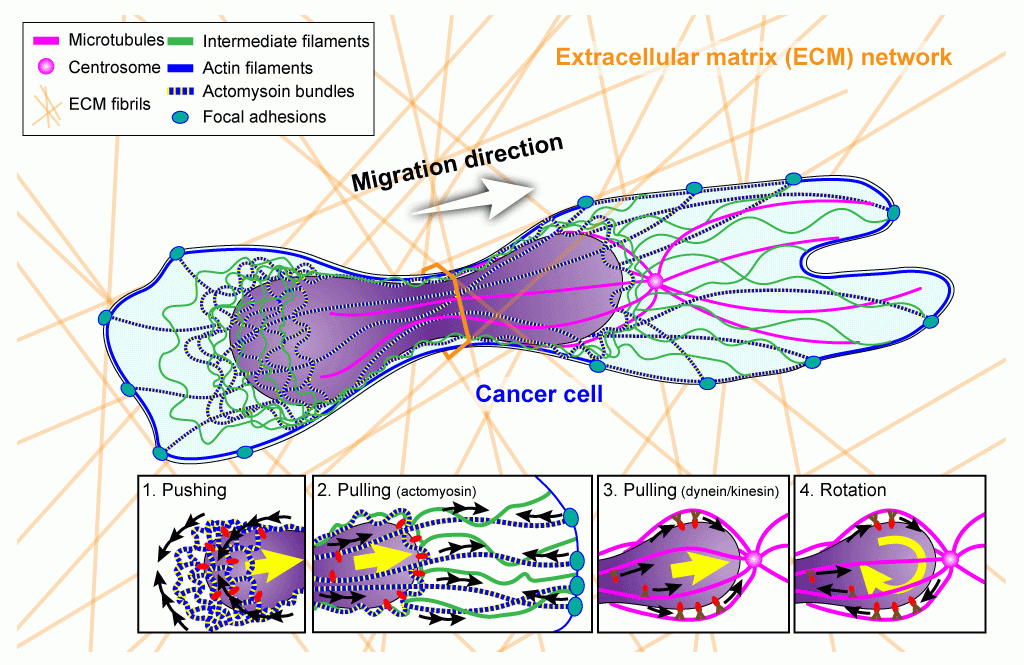
Overview of (proposed) cellular mechanisms to move the large and rigid nucleus through tight spaces during migration in three-dimensional environments such as dense extracellular matrix networks and other interstitial spaces. Image: Jeremiah Hsia, published in Curr Opin Cell Biol 2016 
Time-lapse series of a fibroblast migrating through a 3 µm × 5 µm constriction inside a microfluidic device, revealing extensive deformation of the cell nucleus (red). The cytoskeleton is labeled in green. Image: Patricia Davidson - Elucidating how cells ‘sense’ their physical environment to activate mechanosensitive signaling pathways.
Virtually all cells respond to mechanical stimuli with the activation of specific signaling pathways and induction of mechanosensitive genes. This ‘mechanotransduction’ response enables cells to adjust to their constantly changing physical environment and, for example, drives muscle growth (hypertrophy) in response to exercise, adaptation of bone density to mechanical load, tissue development, and even the interaction/communication between immune cells. While many of the signaling pathways activated by mechanical stimuli are already well characterized, the underlying molecular ‘mechanosensors’ that are responsible for transducing mechanical input into biochemical responses to initiate the specific signaling pathways remain incompletely understood. Recent findings by us and others strongly suggest that the nucleus itself plays a crucial role in the cellular mechanotransduction process. We are using cutting-edge cell and molecular biology approaches combined with novel molecular manipulation and biophysical assays to identify the role of nuclear envelope proteins in mechanotransduction, both in normal cells and when perturbed by disease-causing mutations. By using microfabrication and tissue engineering approaches to create in vitro environments that mimic physiological tissues, we can study the response of individual cells to precisely controlled physical stimuli at high resolution.
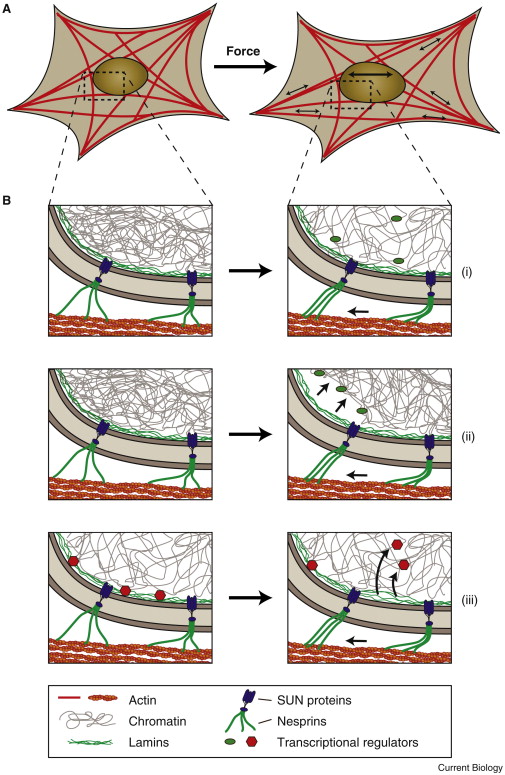
Potential mechanisms of nuclear mechanosensing.Schematic illustration of how force-induced nuclear deformation could modulate expression of mechano-responsive genes. (A) A cell exposed to a uniaxial stretch, resulting in nuclear deformation by forces transmitted from focal adhesions through the (actin) cytoskeleton to the nucleus. (B) Potential molecular mechanisms for nuclear mechanosensing. (i) Opening of chromatin structures under force, enabling access of transcriptional regulators to the chromatin. (ii) Chromatin detachment from the lamina, freeing genes from the often transcriptionally repressive nuclear periphery; this process could also result in further changes in chromatin structure, promoting access to transcriptional regulators. (iii) Stretching the lamina could result in conformational changes or partial unfolding of lamins, altering their interaction with transcriptional regulators. Shown here is the release of transcription factors, which can then interact with their target genes. Phosphorylation and other post-translational modifications of nuclear envelope proteins could further contribute to nuclear mechanosensing. Image: Philipp Isermann, published in Current Biology 2013 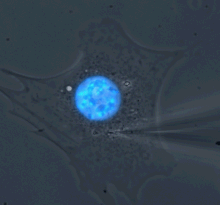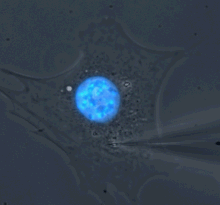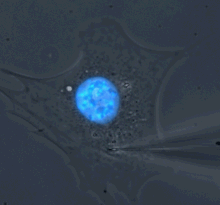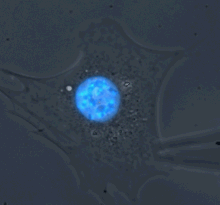
Microharpoon assay to assess intracellular force transmission. Displacement of a fine microneedle inserted into the cytoskeleton induces deformation of the fluorescently labeled nucleus (blue). Image: Greg Fedorchak